Glaucoma Information
Glaucoma Treatment Causes & Symptoms Glaucoma types Harmful drugs Other risks
No Symptoms
 Glaucoma can be difficult to detect without a regular eye exam until a significant amount of vision is lost. The reason it is so dangerous is that most people with glaucoma have no symptoms. Many feel no pain, and most have 20/20 visual acuity, although possibly only straight-ahead vision. But left untreated, glaucoma can slowly steal your peripheral vision until you think you are peering through a tunnel (at best) or until you go blind (at worst). Most frightening, 70% of the vision lost to glaucoma occurs before diagnosis.
Glaucoma can be difficult to detect without a regular eye exam until a significant amount of vision is lost. The reason it is so dangerous is that most people with glaucoma have no symptoms. Many feel no pain, and most have 20/20 visual acuity, although possibly only straight-ahead vision. But left untreated, glaucoma can slowly steal your peripheral vision until you think you are peering through a tunnel (at best) or until you go blind (at worst). Most frightening, 70% of the vision lost to glaucoma occurs before diagnosis.
 Next: Vitamins,
nutrients, diet, & lifestyle tips for glaucoma.
Next: Vitamins,
nutrients, diet, & lifestyle tips for glaucoma.
Damage to the Optic Nerve
The development of glaucoma is brought about by damage to the optic nerve, sometimes as a result of increased pressure in the clear fluid that circulates in the inside of the eye between the cornea and the lens.
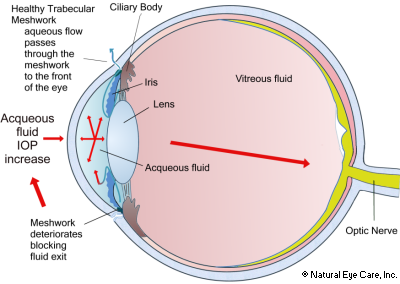
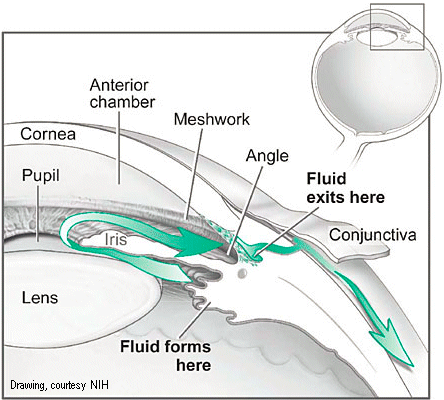
The anterior (front) chamber of the eye is bounded by the lens and iris behind and the cornea in front. The ciliary epithelium behind the upper eyelid secretes aqueous humour, a clear liquid that fills the anterior chamber, which provides oxygen and nutrients to the front of the eye. Aqueous humor is produced continuously and then exits the eyes through a mesh of tiny holes behind the lower eyelid, called the trabecular meshwork.
While it had been logical to assume that increased pressure would restrict the flow of blood to the optic nerve, causing damage, Quigley, et al found (1980) that might not be so.1 Rather, he and his colleagues determined that mechanical compression of individual nerve fiber bundles was a more likely cause.
It is now known that the spongy trabecular meshwork near the lens at the front of the eye becomes clogged and does not permit correct outflow of acqueous humor into the normal body circulation, giving rise to increased intraocular pressure, which swells, sending pressure to the vitreous, and eventually starving the nerve cells of nutrients. The nerves that comprise peripheral vision fail first due to restricted blood flow and/or mechanical compression of nerve fibers.
Furthermore, researchers report that the little cilia, called primary cilia, that extend out from trabecular meshwork cell walls are influenced by biochemicals called phosphoinositides that regulate balance in the aqueous humor.23 If there is damage to these cilia, perhaps due to the meshwork being clogged, then messaging is interrupted and aqueous humor balance is disrupted.
It is further understood that damage caused by elevated eye pressure, the amount of damage, the amount of pressure and the duration of pressure are not perfectly correlated. There may be other factors involved, in part, depending on whether the head of the optic nerve, the ganglion cells and other parts of the optic nerve are damaged and to what extent.2
Glaucoma is now defined as a collection of diseases causing optic nerve damage. The diagnosis is no longer determined only on whether this pressure (Intraocular Pressure or IOL) is high.
Conventional treatment depends on the nature and severity of each case. Commonly, open angle glaucoma is treated with eye drops or oral medications. In the event this treatment does not lower the eye pressure inside the eye, laser therapy or surgery may be considered necessary.
Causes
- Neurodegeneration.
Researchers now view glaucoma as a disease of the brain (a neurodegenerative disease) rather than simply an eye disease. Recent research has shown that the complex connection between the eye and the brain is an important key to the disease. The retina and optic nerve are both made up of brain tissue and are part of the brain. Glaucoma shares a number of features with degenerative brain diseases such as Alzheimer’s, Parkinson’s, and Lou Gehrig’s disease. In these diseases, age and family history are major risk factors, and specific areas of the brain are damaged over time.
In glaucoma, changes occur in the back of the eyes. The optic nerve continues to be a focus for researching the underlying causes of glaucoma. Whether due to mechanical trauma, decreased blood flow, or other causes, optic nerve axon injury causes changes in retinal ganglion cells, eventually resulting in cell death. Researchers have observed that specific areas of injured optic nerve axons and retinal ganglion cell loss match the peripheral vision damage from glaucoma.15 - Neuroinflammation. Glaucoma, especially if acute, may be largely an inflammatory condition. Researchers believe that high intraocular pressure triggers an inflammatory response.16 In experiments, inflammation occurs in the central nervous system and at early stages of glaucoma. Inhibiting the process through which inflammation develops appears to protect the neurons from damage.17 Researchers have been investigating the precise role of neuroinflammation in causing glaucoma. Cells known as microglia behave like sensors to damage the nervous system and play a role in the inflammatory response. The process contributes to beta-amyloid accumulations, implicated in Alzheimer's disease. Similarly, the eye, actually part of the brain, also accumulates beta-amyloid in the retina and optic nerve. Microglia activity and the inflammatory response are linked to protein clumping and nerve cell degeneration.18
- Oxidative stress.
Increased oxidative stress is a risk factor. Antioxidant drugs and nutrients that reduce enzymes involved in oxidation are reported to be helpful in animals with glaucoma. Although targeting IOP has been a prime therapy, researchers are increasingly looking to provide antioxidants for targeting oxidative stress.19 Damage to DNA in the tissue layers that regulate aqueous humor outflow is linked to oxidative damage, as well as damage to neurons and the optic nerve.6,7
Among other results, oxidative stress leads to imbalances in the biochemicals that control relaxation or constriction of blood cells through regulation of the vascular endothelium (a single layer of cells lining blood vessels).13, 14 - Inadequate blood flow due to blood vessel dysfunction at the optic disc (the point where optic nerves exit the eye) 8, or problems in regulation of blood flow to the optic disc.9 Other research finds that problems in blood circulation behind the eyeball contribute to progression of glaucoma.10, 11, 12
- Physical trauma to the eye.
- Harmful drugs. Some medications actually increase your chances of developing glaucoma. Review these harmful drugs for glaucoma.
- Computer use. Heavy computer use increases glaucoma risk. Japanese researchers have found that people who are on the computer all day are at higher risk for developing glaucoma, especially if they are myopic.
- Thyroid. Have your thyroid checked, since some causes of glaucoma have been tied to low thyroid functioning.
- Genetic Mutations. 2009 research found that simultaneous mutations of the WDR36 and STI1 gene are causal factors for glaucoma.
- Mercury, Manganese. 2015 research has identified a connection between high levels of mercury and glaucoma and between low levels and manganese and lower rates of glaucoma.
- Estrogen deficiency. Some research suggests that due to surgery to remove ovaries before menopause, subsequent estrogen deficiencies may increase the risk of developing glaucoma. Women who had the surgery (oophorectomy) before age 43 had a much higher risk of developing glaucoma, even when they were treated with estrogen.3
- Alzheimer's disease. Researchers have found a likely link between Alzheimer's and glaucoma, with Alzheimer's patients being at greater risk of developing glaucoma.4
- Retinal vein occlusion creates an increased risk of developing glaucoma.
- Smoking creates a much larger risk for the user to develop glaucoma. It is true that smoking marijuana descreases intraocular pressure slightly, but only for several hours. Learn more about smoking and glaucoma.
- Mitochondrial dysfunction. Researchers propose that a mitochondrial complex I defect is linked to the deterioration of trabecular meshwork cells, and that antioxidants and MPT (mitochondrial permeability transition inhibitors) can reduce such degeneration.
- Homocysteine. High levels of homocysteine have also been identified as a risk factor for open-angle glaucoma.20 Other studies link high levels of homocysteine to the onset of pseudoexfoliation glaucoma.21,22
Types of Glaucoma
Suspects Open angle Narrow angle Low tension Secondary Congenital

Glaucoma Suspects
A patient deemed a "Glaucoma Suspect" is one typically with a higher than normal eye pressure (IOL), but have not as of yet developed other symptoms such as changes in the optic nerve and/or reduced peripheral vision (as compared to prior visits). Glaucoma suspects could include people with diabetes, hypertension, heart disease, heavy computer users, and people with extreme nearsightedness or farsightedness. They also include people who suffer from obesity, hyperthyroidism, and African Americans. If you are in this category you should have glaucoma testing regularly, and we recommend the nutrients above to protect your vision.
Open Angle or Chronic Glaucoma
Open Angle Glaucoma is most frequently seen. Inside the front of the eye is the trabecular meshwork, which acts as a filter for the fluid in the eye. For various reasons, this meshwork gets clogged or obstructed and does not filter the fluid efficiently, which, in turn, leads to high ocular pressure.
Even though elevated eye pressure is one of the diagnostic tests used for glaucoma, approximately 30-40 percent of patients with open angle glaucoma have normal or low eye pressure and develop optic nerve changes and progressive vision loss without having elevated eye pressure. In the case of this common type of glaucoma there are no symptoms that you will notice until the condition has progressed so that there is loss of peripheral vision. Our complementary protocol is recommended for open angle glaucoma.
Narrow (Acute) Angle Glaucoma or Angle-Closure Glaucoma
This condition is considered an ocular emergency. This results from a blockage to the aqueous fluid draining brought about by a narrow angle between the cornea and the iris that is too narrow to enable the aqueous fluid to drain as quickly as it is being produced. You might experience sudden red eye(s), with headache and visual halos, and sometimes also vomiting and nausea. If you have these symptoms go straight to emergency and call your eye doctor.
Low Tension Glaucoma
Low tension (or normal tension) glaucoma affects 15-25% of patients with glaucoma. It appears with similar problems as open angle glaucoma but the intraocular pressure is in the moderate rather than high range (less than 22 mm Hg). Patients experience the same loss of peripherial vision. It can be caused by an extreme sensitivity of the nerve cells to "normal" intraocular pressure or to blood circulation problems such as vasospasm. The condition is generally an indicator of poor circulation. As a result, not enough oxygen-carrying blood reaches the optic nerve and it becomes damaged.
Many conditions, including brain tumors, heart problems5, or toxic substances can cause optic nerve damage that is experienced as glaucoma. Causes include use of steroids. Other causes include eye trauma, autoimmune disease, thyroid disease, Alzheimer's, sleep apnea, and hypotension (especially in older women). Also see: optic nerve atrophy and optic neuritis.
Low tension glaucoma is more common in Japan and Korea than elsewhere in the world; it affects men more than women; and the mean age is a little older - 60 years old compared to most patients with glaucoma.
Secondary Glaucoma
Secondary Glaucoma arises as a side result to other health conditions such as injury to the eye, inflammation of the eyes, or various drug side effects (such as steroids).
- Exfoliative glaucoma - due to lens deterioration, cell tissue flakes off and becomes clogged in the trabecular meshwork, raising IOP.
- Neovascular glaucoma - because of new blood vessels forming at the front of the eye block fluid from exiting through the trabecular meshwork, raising IOP. These abnormalities are most common in patients who have diabetes
- Pigmentary glaucoma - the iris is composed of particles of pigment. If these detach and float into the vitreous fluid inside the eye they can eventually clog the trabecular meshwork and drainage channels raising IOP.
- Traumatic glaucoma - open angle glaucoma can appear shortly after eye trauma or some time later as debris from the injury disrupts the drainage process.
- Uvetic glaucoma - if the middle layer of the eye, the uvea, becomes inflammed pressure within the eye increases.
- Ghost cell glaucoma - patients who have had vitreous hemorrhage for some time can develop rigid ghost cells which are a type of red blood cell. If these ghost cells get into the front chamber of the eye they can block the trabecular meshwork resulting in ghost cell glaucoma.
Congenital Glaucoma
Congenital Glaucoma is that which begins within the first months after birth. It is the result of incomplete prenatal development of the eye's drainage system. It can also be inherited and can sometimes be treated surgically. Learn more about congenital glaucoma.
Glaucoma News
Want to learn more? See our blog news on glaucoma.
 See
Vitamins & Supplements to support the health of the
optic nerve.
See
Vitamins & Supplements to support the health of the
optic nerve.
Footnotes
1. The mechanism of optic nerve damage in experimental acute intraocular pressure elevation, Quigley, et al
2. Understanding mechanisms of pressure-induced optic nerve damage, Morrison, JD, et al, Oregon Health Sciences University, Portland, Progressive Retina Eye Research. March, 2005, 217-240.
3. The Risk of glaucoma after early bilateral oophorectomy, T.S. Vajaranant, MD., et al, Menopause, April 2014
4. Massimo Cesareo, et al, Association Between Alzheimer's Disease and Glaucoma: A Study Based on Heidelberg Retinal Tomography and Frequency Doubling
Technology Perimetry, Frontiers in Neuroscience, December, 2015.
5. A. J. Lee, et al, Open-angle glaucoma and cardiovascular mortality: the Blue Mountains Eye Study, Ophthalmology, July, 2006
6. Izzotti, A., Bagnis, A., Sacca, S.C. (2006). The role of oxidative stress in glaucoma. Mutat Res, Mar;612(2):105-14.
7. Zhao, J., Wang, S., Zhong, W., Yang, B., Sun, L., et al. (2016). Oxidative stress in the trabecular meshwork. Int J Mol Med, Oct;38(4):995-1002.
8. J. Flammer, S. Orgul, et al, The impact of ocular blood flow in glaucoma, Progressive Retinal Eye Research, 2002
9. F. Galassi, A. Sodi, et al, Ocular Haemodynamics and glaucoma prognosis: a colour Doppler imaging study, Archives of Opthalmology, 2003
10. M. Satilmis, S. Orgul, et al, Rate of progression of glaucoma correlates with retrobulbar circulation and intraocular pressure, American Journal of Ophthalmology, 2003
11. J.J. Zink, J.E. Grunwald, et al, Association between lower optic nerve laser Doppler blood volume measurements and glaucomatous visual field progression, British Journal of Ophthalmology, 2003
12. G. Fuchsjager-Mayrl, B. Wally, et al, Ocular blood flow and system blood pressure in patients with primary open-angle glaucoma and ocular hypertension, Investigations in Ophthalmology and Visual Science, 2004
13. M. Feletou, P.M. Vanhoutte, Endothelial dysfunction: a multifaceted disorder, American Journal of Physiology: Heart and Circulatory Physiology, 2006
14. H. Resch, G. Garhofer, et al, Endothelial dysfunction in glaucoma, Acta Ophthalmologica, June, 2008
15. Sachin, J., Aref, A.A. (2015). Senile Dementia and Glaucoma: Evidence for a Common Link. J Ophthalmic Vis Res, Apr-Jun; 10(2): 178–183.
16. Soto, I., Howell, G.R. (2014). The Complex Role of Neuroinflammation in Glaucoma. Cold Spring Harb Perspect Med, Jul 3;4(8).
17. Ibid. Soto (2014).
18. Ramirez, A.I., de Hoz, R., Salobrar-Garcia, E., Salazar, J.J., Rojas, B., et al. (2017). The Role of Microglia in Retinal Neurodegeneration: Alzheimer's Disease, Parkinson, and Glaucoma. Front Aging Neurosci, Jul 6;9:214.
19. Kimura, A., Namekata, K., Guo, X., Noro, T., Harada, C., et al. (2017). Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis. Oxid Med Cell Longev, 2017:2817252.
20.Roedl, J.B., Bleich, S., Schlotzer-Schrehardt, U., von Ansen, N., ., J., et al. (2008). Increased homocysteine levels in tear fluid of patients with primary open-angle glaucoma. Ophthalmic Res, 40(5):249-56.
21. Bleich, S., Roedl, J., von Ahsen, N., Schlotzer-Schrehardt, U., Reulbach, U., et al. (2004). Elevated homocysteine levels in aqueous humor of patients with pseudoexfoliation glaucoma. Am J Ophthalmol, Jul;138(1):162-4.
22. Leibovitch, I., Kurtz, S., Shemesh, G., Goldstein, M., Sela, B.A., et al. (2003). Hyperhomocystinemia in pseudoexfoliation glaucoma. J Glaucoma, 12 (1), 36-39.
23. Prosseda PP, Alvarado JA, Wang B, Kowal TJ, Ning K, et al. (2020). Optogenetic Stiulation of Phosphoinositides Reveals a Critical
Role of Primary Cilia in Eye Pressure Regulation. Sci Adv. Apr 29;6(18):eaay8699.
Also see Research on glaucoma.
Glaucoma vitamins Causes & Symptoms Treatment Glaucoma types News Other risks
 info@naturaleyecare.com
info@naturaleyecare.com



 Home
Home


 Vision
Vision Vision
Vision



 Health
Health Health
Health Research/Services
Research/Services Pets
Pets About/Contact
About/Contact


